
Scirpus planiculmis extract inhibits the activation of Syk to suppress mast cell-mediated allergic responses in vitro and in vivo
Abstract
Scirpus planiculmis (SP) has been used for a long time as a traditional therapy to cure bronchiectasis, gynecopathy, chest pain, and dyspepsia in Asian countries. However, the therapeutic effect of SP on allergic diseases remains to be determined. In this study, we observed the anti-allergic effect and its mechanism of SP in mast cells and mice. SP significantly suppressed the degranulation and expression of tumor necrosis factor-α (TNF-α) and interleukin-4 (IL-4) in mast cells stimulated by antigen, and its inhibition was reversible. Mechanistically, SP inhibited the activation of spleen tyrosine kinase (Syk) by antigen and its downstream signaling proteins such as linker for activation of T cell (LAT), phospholipase Cγ (PLCγ), Akt, and mitogen activated protein (MAP) kinases, including ERK, JNK, and p38, in a dose-dependent manner. In mice, SP significantly inhibited the passive cutaneous anaphylaxis (PCA) reaction and degranulation of mast cells in ear tissue by antigen. Taken together, SP suppressed the allergic response by antigen in mice via the inhibition of Syk activation in mast cells, which warrants further investigation for developing anti-allergic herbal medicine.
Keywords:
Scirpus planiculmis, Mast cells, Allergy, Passive cutaneous anaphylaxis (PCA), Syk kinaseImmune responses in IgE-mediated hypersensitivity disorders including asthma, atopic dermatitis, and food allergy are exaggerated against innocuous environmental substances.1) The prevalence of allergic disorders has been increasing exponentially around the world for the last few decades.2) The World Allergy Organization analysis indicates that hundreds of millions of people suffer from allergic diseases in countries around the world, and much of the increase is occurring in children.3)
Mast cells are critical for various allergic diseases, especially in IgE-mediated hypersensitive responses. Mast cells are mostly distributed in human tissues adjacent to the external environment, such as airways, internes, and skin.4) When antigen binds to IgE-sensitized mast cells, they rapidly secrete not only allergic mediators including histamine, but also cytokines, such as tumor necrosis factor (TNF)-α, IL-6, IL-13, and interleukin (IL)-4, which eventually leads to late phase allergic responses.5)
When mast cells are activated by antigen, the receptor-proximal Src-family kinases (SFKs) including Lyn and Fyn are initially activated.6) Cytosolic Syk is recruited subsequently to the immunoreceptor tyrosine-based activating motifs (ITAMs) of the FcεRIγ chain, where it becomes activated and stimulates other critical downstream signaling proteins that include linker for activated T cells (LAT) and phospholipase C (PLC) γ for degranulation of mast cells.7) Mitogen-activated protein (MAP) kinases are linked to the production of allergic cytokines and chemokines in mast cells.8) Therefore, the activation of Syk is essential for secretion of histamine, cytokines, and other allergic mediators from mast cells, suggesting that Syk could be an important therapeutic target protein for allergic diseases.
Several types of treatments are used to treat allergic disorders. Pharmaceutical therapies, such as steroid and antihistamine agents, can greatly relieve the symptoms of allergic disease. However, they do not cure the diseases, and can cause unwanted side effects. For example, antihistamine medicine can induce sedation, dizziness, and lack of concentration.9) Long-term administration of steroids can causes various side effects such as Cushing’s syndrome, diabetes, osteoporosis, and decrease in resistance to infection.10) As an alternative approach, allergen-specific immunotherapy has recently been investigated for allergic diseases.11) However, despite the fact that both subcutaneous immunotherapy and sublingual immunotherapy are helpful in reducing allergic symptoms, there are ongoing controversies about the therapeutic efficacy of allergen-specific immunotherapy for allergic diseases.12)
Therefore, many scientists are trying to find alternative therapies, and herbal medicine has been suggested as an option to avoid the severe side effects of drug treatments.13-15) Traditional herbal medicines have a long history, and each country has developed them over many centuries, based on its own traditional usages. East Asian countries, such as Korea and China, have especially tried to find new therapeutic materials from traditional herbal medicines.16-19) Based on such big efforts, some countries including the United States of America have approved several herbal medicines for diseases such as Alzheimer’s, hypercholesterolemia, bowel syndrome, nausea, myocardial infarction, prostatic hyperplasia, and depressive disorder.20,21) The US Food and Drug Administration (FDA) approved VEREGEN® (sinecatechins), a water extract of green tea leaves, as a topical medication for the treatment of external genital and perianal warts in 2006.22,23) FULYZAQ® (MytesiTM, crofelemer), a traditional medicine in the Amazon river area, was approved by the US FDA in 2012 to treat diarrhea.24,25)
In the present study, we found for the first time the inhibitory effect of Scirpus planiculmis (SP) on mast cell-mediated allergic responses in vitro and in vivo. Our observations suggest that SP could be a potential herbal medicine candidate to treat allergic disorders.
Materials and Methods
Reagents - Monoclonal dinitrophenol (DNP)-specific IgE, DNP-bovine serum albumin (BSA), PP2 (a typical tyrosine kinase inhibitor, 1-tert-Butyl-3-(4-chlorophenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine), ionomycin, Evans blue, cetirizine, and toluidine blue were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies for detection of phosphorylation and protein expression of Syk, LAT, PLCγ1, ERK1/2, JNK, p38, and Akt were purchased from Cell Signaling Technology Inc. (Danvers, MA). Cell culture reagents, including minimal essential medium (MEM) with Earle’s salts, Roswell Park Memorial Institute (RPMI) 1640 medium, and fetal bovine serum (FBS), were purchased from GIBCO/Life Technologies Inc. (Rockville, MD).
Plant Extract - The SP extract (specimen number: 037-059) was collected from Wando, Jeonnam in Korea on November 3, 2009, and the extraction and storage of the SP were conducted at the Plant Extract Bank (extract.kribb.re.kr) in the Korea Research Institute of Bioscience and Biotechnology (KRIBB, Daejeon, Korea). It was extracted with methanol using the whole plant and dissolved in dimethyl sulfoxide (DMSO).
Animals - Five-week-old male BALB/c mice were obtained from Orient Bio Experimental Animal Center (Gapyeong, Gyeonggi-do, Korea). Mice were used for isolation of BMMCs and in vivo passive cutaneous anaphylaxis (PCA) study. All animal experiments were conducted in compliance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals after receiving approval from the Institutional Animal Care and Use Committee (IACUC) at Konkuk University (No. KU18127).
Preparation of Mast Cells and Cell Culture - Rat basophilic leukemia (RBL)-2H3 cells were provided by American Type Culture Collection (ATCC) and were grown in MEM supplemented with glutamine, antibiotics and 15% FBS. Bone marrow-derived mast cells (BMMCs) from male BALB/c mice were grown in complete RPMI 1640 medium supplemented with 4 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 25 mM HEPES, 10% FBS, 0.05 mM β-mercaptoethanol and 10 ng/ml IL-3. After 4 - 6 weeks, BMMCs were used for in vitro assays.
Measurement of β-Hexosaminidase Release in RBL-2H3 Cells and BMMCs - Both RBL-2H3 and BMMC cells were primed overnight with 20 ng/ml DNP-specific IgE in 24-well plates (2.0×105 cells/well). Cells were washed twice and resuspended in a final volume of 0.2 ml buffer per well. Buffers used in the assay were as follows : 1,4-piperazinediethanesulfonic acid (PIPES)-buffered medium (25 mM PIPES, pH 7.2, 119 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 1 mM CaCl2, 5.6 mM glucose, and 0.1% fatty acid-free fraction V bovine serum albumin (BSA)) for RBL-2H3 cells and Tyrode buffer (20 mM HEPES, pH 7.4, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% BSA) for BMMCs. Cells were pretreated with or without SP (10, 30, or 100 μg/ml) or PP2 (10 μM) for 30 min, followed by stimulation with 25 ng/ml DNP-BSA for 10 min. Degranulation of mast cells was determined by measuring the ratio of released β-hexosaminidase in supernatants to total β-hexosaminidase in buffer and cell lysates.26)
Western Blot Analysis - Cells were stimulated with 25 ng/ml antigen for the indicated times and chilled on ice to stop the stimulation. Total protein extracts were prepared according to Lee et al..27) In brief, cells were lysed for 30 min in 100 μl lysis buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 10% glycerol, 60 mM octyl β-glucopyranoside, 10 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 2.5 mM p-nitrophenyl phosphate, 0.7 mg/ml pepstatin, and a protease-inhibitor cocktail tablet) on ice. The protein content of cellular extracts was quantified by Bradford assay (absorbance at 595 nm). The lysates were denatured at 100 °C for 5 min in 4× Laemmli buffer. For western blotting, proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories, Berkeley, California). The membranes were blocked in 5% BSA-containing TBS-T buffer for 1 h, and then were incubated with the indicated primary antibodies. The membranes were washed and incubated with horseradish peroxidase-labeled secondary antibodies. Blots were processed using the ImageQuant™LAS 4000 system (GE Healthcare Life Sciences; Piscataway, NJ).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) - Total RNA was prepared from RBL-2H3 cells using the Easy-spinTM Total RNA Extraction Kit (iNtRON Biotechnology, Inc.), and was reverse transcribed using the SuperScript first-strand synthesis system (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. PCR amplification was performed for 30 cycles at 94 °C for 45 s, 55 °C for 45s, and 72 °C for 60 s. The following primers were used: rat TNF-α forward 5’-CACCACGCTCTTCTGTCTACTGAAC-3’; rat TNF-α reverse: 5’-CCGGACTCCGTGATGTCTAAGTACT-3’; rat IL-4 forward 5’-ACCTTGCTGTCACCC TGTTC-3’; rat IL-4 reverse 5’-T TGTGAGCGTGGACTCATTC-3’; rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward 5’-GTGGAGTCTA CTGGCGTCTTC-3’; rat GAPDH reverse 5’-CCAAGGCTGTGGGCAAGGTCA-3’.
Passive Cutaneous Anaphylaxis (PCA) - The procedures for the PCA assay were as previously described.28) In brief, mice were intradermally injected with 0.5 μg DNP-specific IgE into the ear. After 24 h, either SP (100, 300, or 1000 mg/kg) or cetirizine (20 mg/kg) was administered orally to the mice. After 1 h, the mice were challenged with an i.v. injection of 250 mg antigen (DNP-BSA) in 250 ml PBS that contained 4% Evans blue dye (Sigma-Aldrich, MO, USA). After 1 h, the mice were euthanized and the ears were removed to measure the amount of dye present. The dye was extracted from each ear in 700 μl formamide at 63 °C overnight. The absorbance was measured at 620 nm with a microplate reader (Tecan, Männedorf, Switzerland).
Statistical Analysis - The data are expressed as means ± SEM from three or more independent experiments. Statistical analysis was performed using one-way ANOVA and unpaired Student’s t-tests. All statistical calculations were performed using Sigma Stat software (Systat Software, Inc., Point Richmond, CA, USA), with differences considered statistically significant at p < 0.05.
Results
Effect of SP on degranulation in mast cells - The aggregation of high affinity IgE receptors (FcεRI) by antigen releases allergic mediators from cytoplasmic granules in mast cells to induce allergic responses. 8,29) We have screened many plant extracts at a concentration of 100 mg/ml to find herbal anti-allergic medicinal candidates using a degranulation assay in mast cells. Among them, we found that the extract of the whole plant of SP showed the greatest inhibitory effect (approximately 93.7%). To verify further the effect of SP on degranulation by antigen in mast cells, we checked it in RBL-2H3 cells and BMMCs. We observed that the degranulation was inhibited by SP in a dose-dependent manner (IC50, 17.5 mg/ml for RBL-2H3 cells; 23.5 mg/ml for BMMCs) (Fig. 1A). Notably, this inhibitory effect of SP was eliminated by washing the cells after the treatment of SP for 30 min, indicating that the effect of SP was reversible (Fig. 1B).

Scirpus planiculmis (SP) reversibly inhibits degranulation in antigen (Ag)-stimulated mast cells. (A) The amount of β-hexosaminidase released from RBL-2H3 cells and BMMCs was determined. (B) RBL-2H3 cells were washed 3 times after pre-incubation with or without SP (100 μg/ml) or PP2, a typical tyrosine kinase inhibitor, for 30 min. The cells were stimulated with 25 ng/ml Ag for 10 min. β-hexosaminidase release was measured at 450 nm. The values are means ± SEM from three independent experiments. Significant differences compared to Ag-only groups are indicated, *p < 0.05 and **p < 0.01.
Effects of SP on the production of cytokines in mast cells - The stimulation of mast cells by antigen activates the expression and secretion of cytokines, such as IL-4 and TNF-α, which are critical to induction of the late phase allergic responses.30) We thus examined whether SP inhibited the production of cytokines in antigen-stimulated mast cells. Treatment with SP significantly suppressed antigen-stimulated production of TNF-α and IL-4 in a dose-dependent manner (Fig. 2A). The inhibitory potency of SP at 100 mg/ml was almost equivalent to that of PP2, a typical Src-family kinase inhibitor (Fig. 2B).
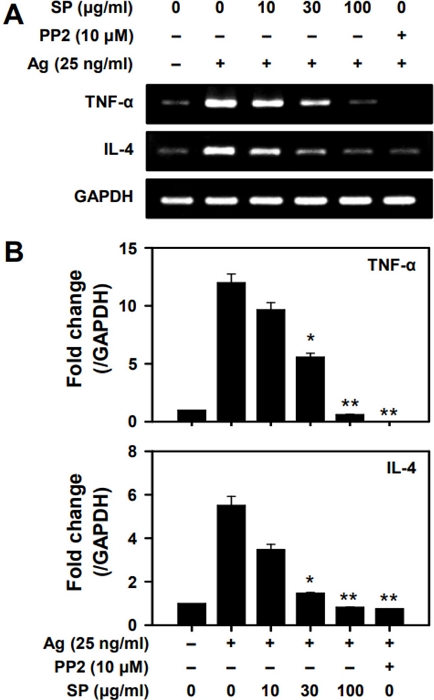
SP inhibits gene expression of TNF-α and IL-4. RT-PCR was performed for (A) TNF-α and IL-4. (B) Densitometric values (mean ± SEM) for three independent experiments are shown. Significant differences compared to Ag-only groups are indicated, *p < 0.05 and **p < 0.01.
Effect of SP on the activation of Syk and its downstream signaling proteins by antigen in mast cells - The aggregation of FcεRI’s by antigen activates Syk kinase, an essential signaling protein for the activation of mast cells, subsequently activating many downstream signaling proteins.31,32) Our results show that SP significantly inhibited activations of Syk, LAT, PLCγ1, and Akt by antigen in RBL-2H3 cells and in BMMCs (Fig. 3). MAP kinases (e.g., ERK1/2, JNK, and p38) are critical for the production of TNF-α and IL-4.33) The activations of ERK1/2, JNK, and p38 were suppressed by SP in a dose-dependent manner (Fig. 3B). These results indicate that SP suppresses mast cells via inhibition of activation of Syk and the Syk-dependent proteins, LAT, PLCγ1, Akt, and MAP kinases.
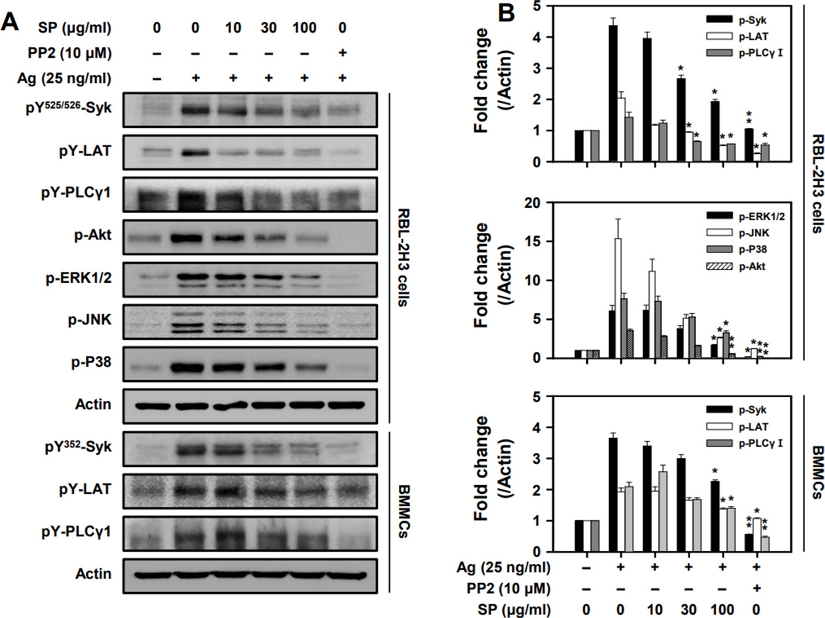
SP inhibits phosphorylation of Syk and downstream molecules in mast cells. Representative (A) immunoblotting images and (B) band densities for phosphorylation of signaling proteins. The cell lysates were subjected to western blot analysis. Significant differences compared to Ag-only groups are indicated, *p < 0.05 and **p < 0.01.
Effect of SP on antigen-induced passive cutaneous anaphylaxis (PCA) in mice - We used PCA mouse to measure the anti-allergic effect of SP in vivo. PCA has been reported as a mast cell-mediated allergic mouse model.16,34) In our study, the PCA reaction was suppressed by SP in a dose-dependent manner (Fig. 4A and B). This inhibitory potency of SP at a dose of 1,000 mg/kg was similar to that of cetirizine (20 mg/kg), an anti-allergic medicine, suggesting that SP could also be used as an anti-allergic herbal medicine. In histology examinations, we further observed that SP also inhibited degranulation of mast cells in the ear tissues of antigen-induced PCA mice (Fig. 4C and D).
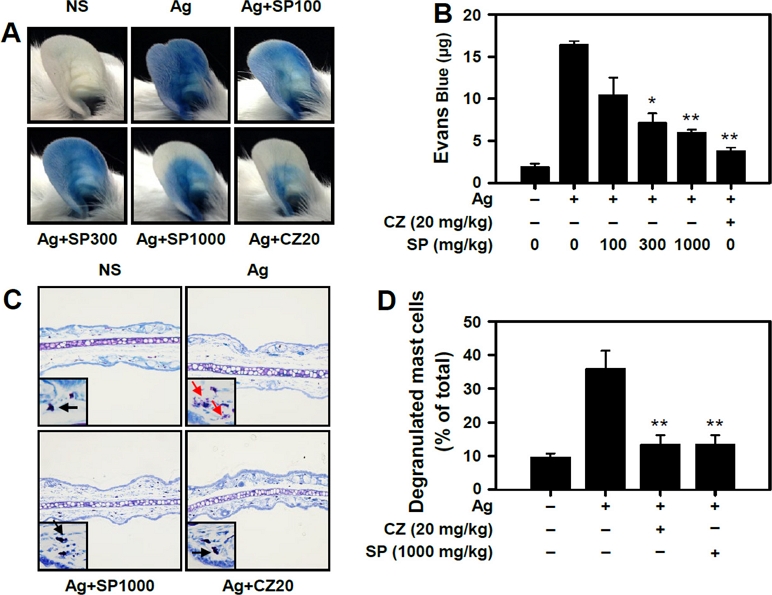
SP inhibits the passive cutaneous anaphylaxis (PCA) reaction and degranulation of mast cells in vivo. Mice were intradermally injected with DNP-specific IgE (50 ng) in their ear. After 12 hours, they were orally administered with SP or cetirizine serving as a reference drug. One hour later, DNP-HSA with Evans Blue was administered by intravenous injection. (A) Representative photographs of ears and (B) quantification of extracted Evans blue dye. (C) Representative images for histological examination of ears. Black arrow, resting mast cells; Red arrow, degranulated mast cells. (D) Percentage of degranulated-mast cells was measured in the ear. Data are presented as the mean ± SEM obtained from 3 individual experiments. n = 5 mice per group. Significant differences compared to Ag-only groups in degranulation are indicated, *p < 0.05. and **p < 0.01. CZ, cetirizine.
Discussion
Although the number of allergic patients has increased in past decades, current therapeutic approaches are not ideal. Recently, alternative and complementary herbal medicines have been suggested as additional therapeutic options for allergic disorders. Particularly, some traditional herbal extracts offer important advantages for drug development due to their low side effects. For example, the herbal extract medicines, Stillen (Donga Pharmaceutical Co., Ltd., Korea) and Joins (SK Chemicals, Korea), have been used as treatments for gastric ulcers and arthritis in the market, respectively.
SP, which belongs to the Poales order and Cyperaceae family, mainly grows in coastal wetlands; SP is widely found in Northeast Asia, including Korea, Japan, and China. SP has long been used to treat bronchiectasis, dysmenorrhea, chest pain, and dyspepsia as traditional folk remedies.35,36) However, its pharmacological effects and its mechanisms of action, especially on allergic responses, have not been investigated.
The activation of IgE-labeled mast cells by antigen plays a pivotal role to induce allergic responses. When mast cells are stimulated by antigen, the cells rapidly release stored chemical mediators within cytosolic granules, including histamines, tryptases, chemokines, and pro-inflammatory cytokines, which leads to allergic responses.5) Our results showed that SP inhibited degranulation of mast cells by antigen in vitro and also suppressed allergic response in PCA mice (Fig. 1 and 4). Notably, the inhibition by SP of mast cell degranulation was reversible (Fig. 1B). Based on these results, SP could be a good candidate as an anti-allergic herbal medicine.
When mast cells are stimulated with antigen, Src family kinases (SFKs) are initially activated. Syk is then activated, followed by LAT, PI3K/Akt, and MAP kinase activation, and the mobilization of intracellular Ca2+, to stimulate degranulation and the release of cytokines and another allergic mediators, eicosanoids.31,33,34,37) Therefore, Syk inhibitors could be good therapeutic approaches to treat allergic disorders.38,39) In this study, SP inhibited activation of Syk in antigen-stimulated mast cells (Fig. 3), suggesting that SP exerts its anti-allergic effect in vitro and in vivo through suppressing Syk in mast cells. Upon antigen stimulation, Syk undergoes activation through two distinct phosphorylation mechanisms One mechanism is that Syk is phosphorylated and activated by upstream Src-family kinases such as Lyn or Fyn. Another mechanism is the Syk's auto-phosphorylation. Therefore, further studies are needed to determine by which of these two mechanisms SP inhibits to suppress the phosphorylation of Syk.
The activations of MAP kinases, such as ERK, JNK, and p38, are essential for the production of cytokines.8,37,40-43) The cytokines (e.g., TNF-α and IL-4) are very closely associated with the pathogenesis of allergic inflammation.44,45) SP suppressed expression levels of TNF-α and IL-4 (Fig. 2) and activation of the three MAP kinases, ERK1/2, JNK, and p38 (Fig. 3). These results suggest that SP can suppress cytokine-mediated late-phase allergic responses in the progression of allergic diseases by inhibiting MAP kinases.
In humans, when an allergen enters the body, B cells produce IgE under atopic conditions. The IgEs subsequently bind to FcεRI receptors on mast cells located in peripheral tissues. The same allergen then binds to FcεRI on mast cells, inducing the secretion of granules and allergic mediators. PCA has been used as a standard model for mast cell-mediated type I hypersensitivity reaction in mice.46) In our results, SP effectively suppressed the PCA reaction in a dose-dependent manner (Fig. 4), suggesting that SP has an inhibitory effect on allergic responses by suppressing mast cell degranulation in vivo.
Conclusions
We observed for the first time that SP inhibited degranulation and allergic cytokine production (i.e., TNF-α and IL-4) in mast cells in vitro and in vivo by suppressing activation of Syk and Syk-mediated signaling cascade (Fig. 5). Although further research is needed to identify which substances within the extract exhibit anti-allergic activity, our results suggest that SP could be useful for IgE-mediated allergic diseases such as allergic rhinitis, asthma, and atopic dermatitis.
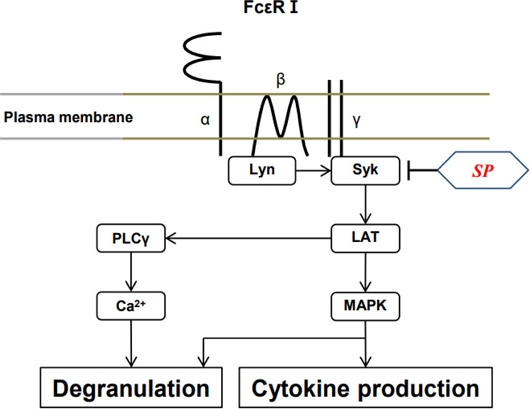
A hypothetical scheme for the mechanism by which SP inhibits the activation of mast cells. Phosphorylation of Syk by Ag stimulation leads to activation of downstream signaling molecules, such as LAT, PLC-γ, leading to Ca2+ mobilization, and MAP kinases. The inhibitory effect of SP on mast cell activation is due to the inhibition of Syk activation.
Acknowledgments
This study was supported by Duksung Women’s University in 2023. We would also like to express our gratitude to Professor Wahn Soo Choi and Dr. Hyunwoo Kim from the College of Medicine at Konkuk University for their invaluable comments and support for this paper.
References
- Gotua, M., Lomidze, N., Dolidze, N. and Gotua, T. (2008) IgE-mediated food hypersensitivity disorders. Georgian Med. News 157: 39-44.
-
Asher, M. I., Montefort, S., Bjorksten, B., Lai, C. K., Strachan, D. P., Weiland, S. K., Williams, H. (2006) Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 368: 733-743.
[https://doi.org/10.1016/S0140-6736(06)69283-0]

- Pawanker, R., Canonica, G. W., Holgate, S. T. and Lockey, R. F. (2011) World Allergy Organization (WAO) White Book on Allergy.
-
Urb, M. and Sheppard, D. C. (2012) The role of mast cells in the defense against pathogens. PLoS Pathog. 8: e1002619.
[https://doi.org/10.1371/journal.ppat.1002619]

-
Metcalfe, D. D., Baram, D. and Mekori, Y. A. (1997) Mast cells. Physiol. Rev. 77: 1033–1079.
[https://doi.org/10.1152/physrev.1997.77.4.1033]

-
Gilfillan, A. M. and Beaven, M. A. (2011) Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 31: 475–530.
[https://doi.org/10.1615/CritRevImmunol.v31.i6.30]

-
Gilfillan, A. M. and Rivera, J. (2009) The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 228: 149–169.
[https://doi.org/10.1111/j.1600-065X.2008.00742.x]

-
Gilfillan, A. M. and Tkaczyk, C. (2006) Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 6: 218–230.
[https://doi.org/10.1038/nri1782]

-
Ridolo, E., Montagni, M., Bonzano, L., Incorvaia, C. and Canonica, G. W. (2015) Bilastine: new insight into antihistamine treatment. Clin. Mol. Allergy 13: 1.
[https://doi.org/10.1186/s12948-015-0008-x]

-
Hengge, U. R., Ruzicka, T., Schwarts, R. A. and Cork, M. J. (2006) Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 54: 1-15.
[https://doi.org/10.1016/j.jaad.2005.01.010]

-
Akdis, M. and Akdis, C. A. (2007) Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 119: 780-791.
[https://doi.org/10.1016/j.jaci.2007.01.022]

-
Yukselen, A. and Kendirli, S. G. (2014) Role of immunotherapy in the treatment of allergic asthma. World J. Clin. Cases 2: 859-865.
[https://doi.org/10.12998/wjcc.v2.i12.859]

-
Ernst, E. (2011) Herbal medicine in the treatment of rheumatic diseases. Rheum Dis. Clin. North Am. 37: 95-102.
[https://doi.org/10.1016/j.rdc.2010.11.004]

-
Lee, J. H., Kim, J. W., Ko, N. Y., Mun, S. H., Her, E., Kim, B. K., Han, J. W., Lee, H. Y., Beaven, M. A., Kim, Y. M. and Choi, W. S. (2008) Curcumin, a constituent of curry, suppresses IgE-mediated allergic response and mast cell activation at the level of Syk. J. Allergy Clin. Immunol. 121: 1225-1231.
[https://doi.org/10.1016/j.jaci.2007.12.1160]

-
Yang, X. X., Hu, Z. P., Duan, W., Zhu, Y. Z. and Zhou, S. F. (2006) Drug-herb interactions: eliminating toxicity with hard drug design. Curr. Pharm. Des. 12: 4649-4664.
[https://doi.org/10.2174/138161206779010440]

-
Kim, J. W., Lee, J. H., Hwang, B. Y., Mun, S. H., Ko, N. Y., Kim, D. K., Kim, B., Kim, H. S., Kim, Y. M. and Choi, W. S. (2009) Morin inhibits Fyn kinase in mast cells and IgE-mediated type I hyperpersensitivity response in vivo. Biochem. Pharmacol. 77: 1506–1512.
[https://doi.org/10.1016/j.bcp.2009.01.019]

-
Lee, J. H., Kim, J. W., Ko, N. Y., Mun, S. H., Kim, D. K., Kim, J. D., Won, H. S., Shin, H. S., Kim, H. S., Her, E., and Choi, W. S. (2008) Mast cell-mediated allergic response is suppressed by Sophorae flos: inhibition of SRC-family kinase. Exp. Biol. Med. 233: 1271-1279.
[https://doi.org/10.3181/0803-RM-89]

-
Gu, S., Yang, A. W., Xue, C. C., Li, C. G., Pang, C., Zhang, W. and Williams, H. C. (2013) Chinese herbal medicine for atopic eczema. Cochrane Database Syst. Rev. 9: CD008642.
[https://doi.org/10.1002/14651858.CD008642.pub2]

-
Kim, T. H., Cho, K. H., Jung, W. S. and Lee, M. S. (2012) Herbal medicines for Parkinson’s disease: a systematic review of randomized controlled trials. PLoS One 7: e35695.
[https://doi.org/10.1371/journal.pone.0035695]

-
Benzie, I. F. and Wachtel-Galor, S. (2011) Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition, CRC Press, Boca Raton (FL), Chapter 1, http://www.ncbi.nlm.nih.gov/books/NBK92773/, .
[https://doi.org/10.1201/b10787]

-
Bent, S. (2008) Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J. Gen. Intern. Med. 23: 854-859.
[https://doi.org/10.1007/s11606-008-0632-y]

- Kelly, P. (2009) Sinecatechins (Veregen) for external genital and perianal warts. Am. Fam. Physician. 80: 1447-1454.
-
Fürst, R. and Zündorf, I. (2014) Plant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediators inflamm. 2014:146832.
[https://doi.org/10.1155/2014/146832]

- FDA (2012) Safety and Effectiveness of 3 Doses of Crofelemer Compared to Placebo in the Treatment of HIV Associated Diarrhea (ADVENT), Clinical trial number NCT00547898.
- Mader, L. S. (2012) FDA delays decision on crofelemer for second time. HerbalEGram 9 http://cms.herbalgram.org/heg/volume9/11November/Crofelemer_2ndFDAdelay.html, .
-
Ozawa, K., Szallasi, Z., Kazanietz, M. G., Blumberg, P. M., Mischak, H., Mushinski, J. F. and Beaven, M. A. (1993) Ca(2+)-dependent and Ca(2+)-independent isozymes of protein kinase C mediate exocytosis in Antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J. Biol. Chem. 268: 1749–1756.
[https://doi.org/10.1016/S0021-9258(18)53916-8]

-
Lee, J. H., Kim, Y. M., Kim, N. W,. Kim, J. W., Her, E., Kim, B. K., Kim, J. H., Ryu, S. H., Park, J. W., Seo, D. W. and Choi, W. S. (2006) Phospholipase D2 acts as an essential adaptor protein in the activation of Syk in antigen-stimulated mast cells. Blood 108: 956-964.
[https://doi.org/10.1182/blood-2005-10-009159]

-
Lee, J. H., Kim, J. W., Ko, N. Y., Mun, S. H., Kim, D. K., Kim, J. D., Kim, H. S., Lee, K. R., Kim, Y. K., Radinger, M., and Choi W. S. (2008) Camellia japonica suppresses immunoglobulin E-mediated allergic response by the inhibition of Syk kinase activation in mast cells. Clin. Exp. Allergy 38: 794-804.
[https://doi.org/10.1111/j.1365-2222.2008.02936.x]

-
Rivera, J., Fierro, N.A., Olivera, A. and Suzuki, R. (2008) New insights on mast cell activation via the high affinity receptor for IgE. Adv. Immunol. 98: 85-120.
[https://doi.org/10.1016/S0065-2776(08)00403-3]

-
Bradding, P., Roberts, J. A., Britten, K. M., Montefort, S., Djukanovic, R., Mueller, R., Heusser, C. H., Howarth, P. H. and Holgate, S. T. (1994) Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am. J. Respir. Cell Mol. Biol. 10: 471-480.
[https://doi.org/10.1165/ajrcmb.10.5.8179909]

-
Siraganian, R. P., Zhang, J., Suzuki, K. and Sada, K. (2002) Protein tyrosine kinase Syk in mast cell signaling. Mol. Immunol. 38: 1229–1233.
[https://doi.org/10.1016/S0161-5890(02)00068-8]

-
Siraganian, R. P., de Castro, R. O., Barbu, E. A. and Zhang, J. (2010) Mast cell: The role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 584: 4933-4940.
[https://doi.org/10.1016/j.febslet.2010.08.006]

-
Rivera, J. and Gilfillan, A. M. (2006) Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 117: 1214–1225.
[https://doi.org/10.1016/j.jaci.2006.04.015]

-
Kabu, K., Yamasaki, S., Kamimura, D., Ito, Y., Hasegawa, A., Sato, E., Kitamura, H., Nishida, K. and Hirano, T. (2006) Zinc is required for FcεRI-mediated mast cell activation. J. Immunol. 177: 1296–1305.
[https://doi.org/10.4049/jimmunol.177.2.1296]

-
Jung, J. and Choi, H. K. (2013) Recognition of two major clades and early diverged groups within the subfamily Cyperoideae (Cyperaceae) including Korean sedges. J. Plant Res. 126: 335-349.
[https://doi.org/10.1007/s10265-012-0534-2]

- Naczi, R. F. C. and Ford, B. A. (2008) Sedges: uses, diversity and systematics of the Cyperaceae. Missouri Botanical Garden Press. St. Louis (MO), 108: 298.
-
Frossi, B., Rivera, J., Hirsch, E. and Pucillo, C. (2007) Selective activation of Fyn/PI3K and p38 MAPK regulates IL-4 production in BMMC under nontoxic stress condition. J. Immunol. 178: 2549-2555.
[https://doi.org/10.4049/jimmunol.178.4.2549]

-
Suljagic, M., Longo, P. G., Bennardo, S., Perlas, E., Leone, G., Laurenti, L. and Efremov, D. G. (2010) The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Eμ-TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood 116: 4894-4905.
[https://doi.org/10.1182/blood-2010-03-275180]

-
Spurgeon, S. E., Coffey, G., Fletcher, L. B., Burke, R., Tyner, J. W., Druker, B. J., Betz, A., DeGuzman, F., Pak, Y., and Baker, D., Pandey, A., Hollenbach, S.j., Sinha, U. and Loriaux, M.M. (2013) The selective Syk inhibitor P505-15 (PRT062607) inhibits B cell signaling and function in vitro and in vivo and augments the activity of Fludarabine in chronic lymphocytic leukemia. J. Pharmacol. Exp. Ther. 344: 378-387.
[https://doi.org/10.1124/jpet.112.200832]

-
Lorentz, A., Klopp, I., Gebhardt, T., Mann,s M. P. and Bischoff, S. C. (2003) Role of activator protein 1, nuclear factor-kappaB, and nuclear factor of activated T cells in IgE receptor-mediated cytokine expression in mature human mast cells. J. Allergy Clin. Immunol. 111: 1062-1068.
[https://doi.org/10.1067/mai.2003.1342]

-
Beyaert, R., Cuenda, A., Berghe, W. V., Plaisance, S., Lee, J. C., Haegeman, G., Cohen, P. and Fiers, W. (1996) The P38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 15: 1914-1923.
[https://doi.org/10.1002/j.1460-2075.1996.tb00542.x]

-
Zhang, C., Baumqartner, R. A., Yamada, K. and Beave,n M. A. (1997) Mitogen-activated protein (MAP) kinase regulates production of tumor necrosis factor-alpha and release of arachidonic acid in mast cells. J. Biol. Chem. 272: 13397-13402.
[https://doi.org/10.1074/jbc.272.20.13397]

-
Ishizuka, T., Terada, N., Gerwins, P., Hamelmann, E., Oshiba, A., Fanger, G. R., Johnson, G. L. and Gelfand, E. W. (1997) Mast cell tumor necrosis factor alpha production is regulated by MEK kinases. Proc. Natl. Acad. Sci. U S A. 94: 6358-6363.
[https://doi.org/10.1073/pnas.94.12.6358]

-
Bischoff, S. C. (2007) Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat. Rev. Immunol. 7: 93–104.
[https://doi.org/10.1038/nri2018]

-
Supajatura, V., Ushio, H., Nakao, A., Akira, S., Okumura, K., Ra, C. and Ogawa, H. (2002) Differential responses of mast cell toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Invest. 109: 1351–1359.
[https://doi.org/10.1172/JCI0214704]

-
Lee, J. K. and Vadas, P. (2011) Anaphylaxis: mechanisms and management. Clin. Exp. Allergy 41: 923-938.
[https://doi.org/10.1111/j.1365-2222.2011.03779.x]
